MATTER,ATOMS,CHEMICAL REACTIONS.
Chemistry is the study of matter, and all matter is made up of atoms.
In this lesson we will learn about elements, atomic number and mass, isotopes, and compounds.
Introduction to the atom
In chemistry, we will often be thinking about the world on a much smaller scale than you can see with the naked eye. Here we will learn about atoms (tiny particles that are around us) and elements. What are atoms, and what kind of properties do they have? How do we weigh and count atoms? We will answer those questions in this section!
ELEMENTS
The human body contains 65% oxygen, 18% carbon, 10% hydrogen, 3% nitrogen, 1.5% calcium, 1% phosphorus, 0.35% potassium, 0.25% sulfur, 0.15% sodium, 0.05% magnesium, and copper, zinc, selenium, molybdenum, fluorine, chlorine, iodine, manganese, cobalt, iron have a percentage of about 0.7%, and there are trace amounts of lithium, Strontium, aluminum, silicon, lead, vanadium, arsenic, bromine are in trace amounts.
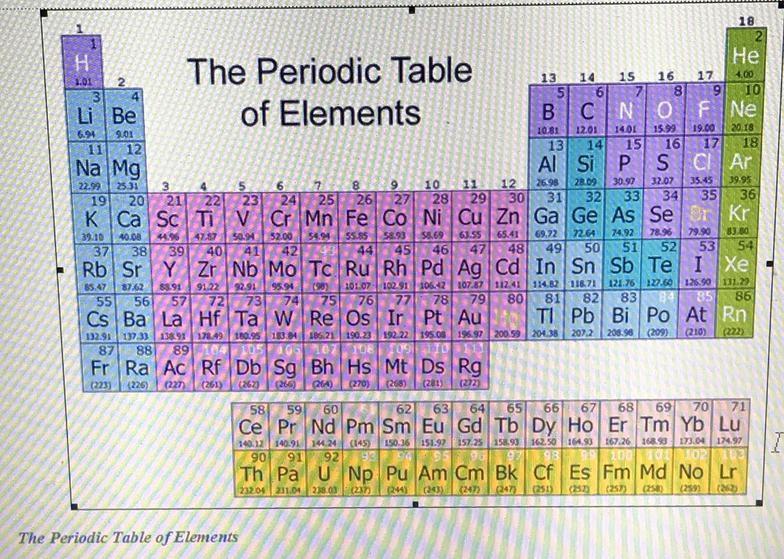
Atomic Number
The atomic number tells you how many protons and neutrons atom has. For example, every hydrogen atom has the atomic number 1 because it only has 1 proton.
Elements that have atomic numbers of up to 92 can be found in nature; those over 92 are created by scientists in a laboratory.
The atomic number tells us where we can find an element in the periodic table. This table shows all the atoms in groups.
Atomic Mass
The atomic mass is the number of protons and neutrons in an atom. Although all atoms of the same element have the same number of protons, they sometimes have more neutrons. Such atoms are called isotopes.
For example, hydrogen has three isotopes. Most of the time a hydrogen atom has one proton and one neutron. Sometimes you can find hydrogen isotopes that have two or three neutrons, but they too have only one proton.
In most lighter elements the nucleus of each atom has the same number of protons and neutrons. but heavier elements have more neutrons than protons. Uranium , for example has 92 protons and 146 neutrons. It’s atomic mass is 238.
The atomic mass is never a whole number, because scientists do not just add protons and neutrons together. They use a complicated formula.